Adverse Event Management Software
Turn Adverse Events into Drivers of Change and Quality
Navigating the complexity of adverse event management is more than complying with stringent regulations; it’s about safeguarding what matters most—patients, customers, and product integrity. By adopting a next-generation Adverse Events Management System like Qualityze, organizations can efficiently capture, report, and resolve adverse events while ensuring compliance with evolving regulatory standards. Seamlessly integrating data, insights, and workflows, this approach helps you stay a step ahead in the journey from incident to improvement.
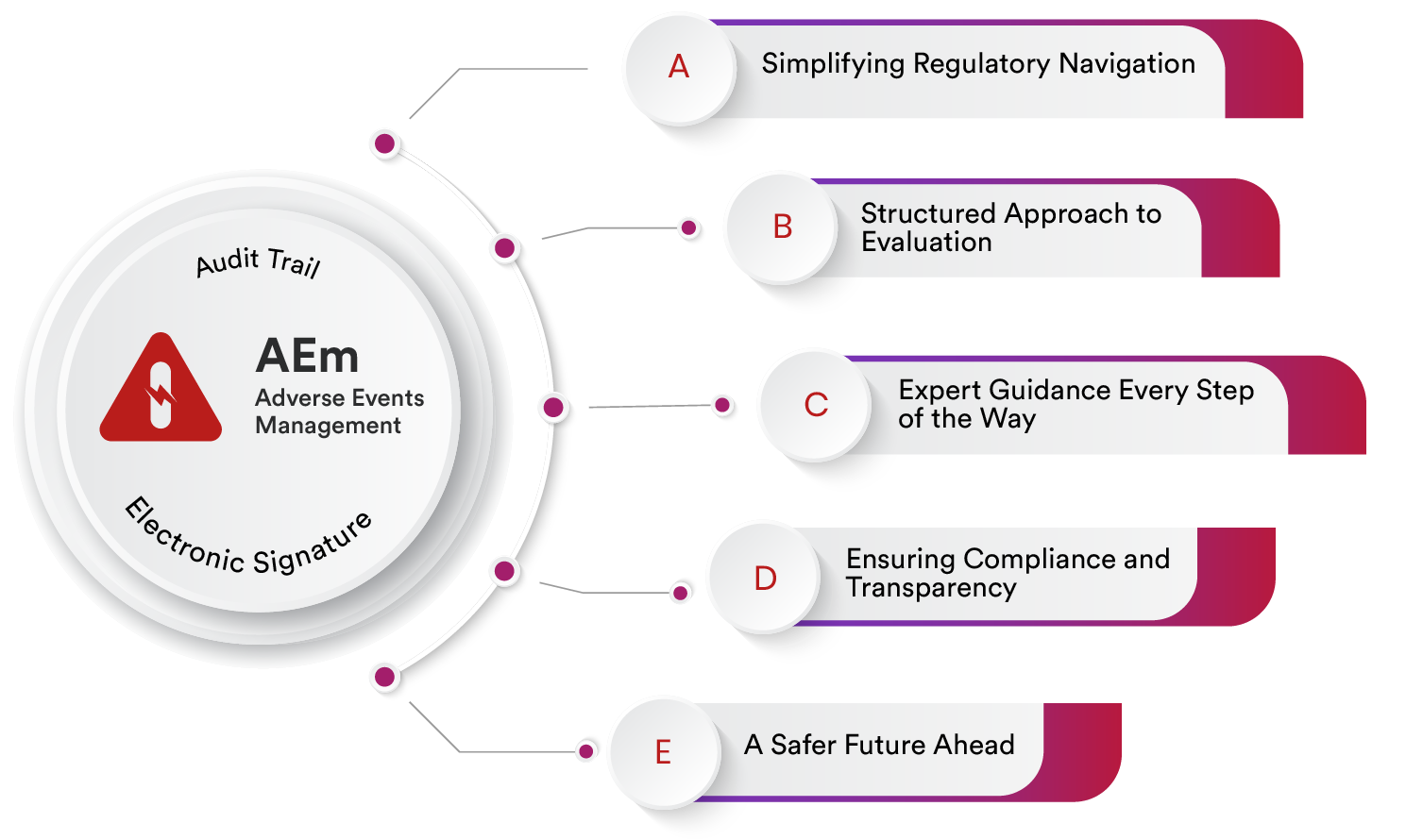

AI Powered Adverse Events Management for Patient Safety and Compliance Confidence
Qualityze AI Powered Adverse Event and Complaint Management Software enable organizations to systematically evaluate events based on severity and regulatory criteria for each region. With built-in guidance for every step, from evaluation to reporting, Qualityze ensures your team stays compliant, informed, and prepared, promoting a culture of transparency, accountability, and patient safety at all levels.

Simplify Regulatory Navigation for Adverse Events
Qualityze's intuitive interface takes the guesswork out of regulatory reporting by streamlining the identification of adverse events across your organization. By capturing all necessary details, such as event type, source, and potential impact, and storing them in a centralized database, the software helps ensure a comprehensive understanding of diverse regulatory requirements across different regions or countries. This empowers your organization to make informed decisions regarding the necessity of reporting based on the specific context of each event.

Structured Evaluation for Adverse Event Reporting
With Qualityze, you can establish a systematic method to evaluate reportable events by considering key factors such as severity, likelihood, and regional obligations. This structured evaluation process ensures consistent assessment of each event and helps prioritize those that require immediate reporting. Configurable workflows enable your team to easily update evaluation criteria as new information becomes available, maintaining compliance while keeping your adverse event management agile and responsive.

Set Thresholds for Reportable Events
Defining clear evaluation criterias for adverse events helps determine which events warrant regulatory reporting. Qualityze allows you to set these criterias, flagging events that matches with these criteria for immediate action. This proactive approach ensures that critical events are addressed promptly, supporting adherence to industry regulations and demonstrating a commitment to transparency and patient safety.

Conduct Preliminary Impact Assessments for Adverse Events
Qualityze facilitates preliminary impact assessments for each identified adverse event, allowing you to compare both severity and likelihood. By categorizing events across five levels of impact—Insignificant, Minor, Moderate, Major, and Catastrophic—Qualityze ensures a structured approach that prioritizes the most critical events, enabling your organization to take timely and effective action to mitigate risks.

Serious Event Escalation and Automated Notification
For adverse events classified as serious, Qualityze provides automated flagging based on predefined criteria such as death, hospitalization, or life-threatening conditions. Immediate notifications are sent to relevant personnel, such as the safety team or medical director, ensuring prompt review and action.

Regulatory and Internal Reporting for Compliance
Qualityze streamlines regulatory reporting by generating standardized reports for submission to regulatory agencies like the FDA, EMA, TGA, Health Canada, PMDA and others within specified timelines. The system also supports internal reporting, disseminating information to internal stakeholders such as safety committees and clinical teams, enabling analysis and decision-making in a timely manner.

Signal Detection and Ongoing Monitoring
Qualityze supports signal detection through advanced data analysis, using statistical methods to identify potential safety signals and emerging patterns of adverse events. The software also facilitates ongoing monitoring, enabling periodic reviews of adverse event data to identify trends and emerging safety concerns. Product safety updates can be communicated to healthcare providers and patients as needed.

Integrate with Enterprise Quality Systems for Comprehensive Adverse Event Control
Qualityze Adverse Event Management integrates seamlessly with other quality systems such as Complaint Management, CAPA, and Change Management. This integration enables a unified approach to managing adverse events, supporting proactive mitigation, and driving continuous improvement across all quality functions. By fostering an interconnected system, Qualityze helps enhance transparency, compliance, and operational efficiency, ultimately promoting a safer environment for patients.

Integrate with FDA eMDR system
Qualityze Adverse Event Management seamlessly integrates with the FDA eMDR system, streamlining the adverse event reporting process. It automates submissions in HL7 ICSR XML format, tracks the status of reports in real-time (including Ack1, Ack2, and Ack3 acknowledgments), and ensures compliance with FDA regulations.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
Adverse events are harmful or undesirable effects of medical treatment. Adverse events negatively impact the patient care, outcomes, and healthcare costs. In a paper—Estimating the Cost of Adverse Events in Economic Model –. published by the US Institute of Medicine of the National Academy of Sciences, six categories of waste were held responsible for driving healthcare inflation in the US.
We have got you covered for everything you look for in a next generation Risk management solution to be future-ready!
- End-to-End Adverse Event Lifecycle Management
Qualityze Adverse Event Management offers complete oversight of the entire adverse event lifecycle—from capturing initial reports to investigation, evaluation, corrective action, and ongoing monitoring. Every adverse event, regardless of its severity, is effectively managed, ensuring patient safety and regulatory compliance.
- Configurable to Fit Your Needs
Qualityze is designed to adapt to your organization's needs. You can easily configure fields, workflows, and templates to match your specific adverse event reporting and management requirements, ensuring that your processes are both effective and aligned with industry standards.
- Built-In Compliance Tools
Stay ahead of evolving regulatory requirements with built-in compliance tools. Qualityze streamlines adverse event reporting with real-time regulatory updates, automated severity assessments, and pre-configured workflows, ensuring your organization remains audit-ready and compliant with all relevant regulations.
- Seamless Integration
Qualityze integrates seamlessly with existing quality management systems, such as CAPA, Complaint Management, and ERP systems. This interconnected ecosystem provides a unified platform for managing adverse events, eliminating silos, and promoting greater efficiency across your organization.
- User-Friendly Interface
Qualityze Adverse Event Reporting features an intuitive, cloud-based interface that requires minimal training. Whether your team is in the office or working remotely, our user-friendly platform makes it easy to capture, document, and manage adverse events efficiently. Advanced search tools ensure that users can quickly access the information they need, streamlining event tracking and resolution.
- Continuous Improvement Culture
Qualityze promotes a culture of continuous improvement by providing a closed-loop system for managing adverse events. It empowers your team to evaluate incident trends, implement corrective actions, and proactively enhance safety measures. By consistently monitoring and improving the adverse event reporting process, Qualityze helps ensure that your organization is resilient and prepared for future challenges.
- Unparalleled Support and Training
Our dedicated support team provides personalized training, ongoing assistance, and regular updates to ensure your adverse event reporting processes remain efficient and compliant. Qualityze empowers your organization to continuously improve and adapt to evolving regulatory requirements, fostering a proactive approach to quality and safety management.
By leveraging Qualityze Risk Management, organizations can achieve higher levels of quality while effectively managing costs, resulting in improved customer satisfaction, strengthened brand reputation, and increased profitability.
Industry Recognitions



Risk management benefits to various operational functions
Discover how Qualityze Adverse Event Management helps different roles manage adverse events more proactively and efficiently using advanced built-in capabilities—from Quality Assurance Managers to Legal Specialists.
For Quality Managers
Quality Managers play a crucial role in ensuring that adverse events are managed in alignment with quality standards. Qualityze provides tools to capture adverse events, conduct root cause analysis, and implement corrective actions promptly. This helps maintain product quality, prevent recurrence of incidents, and enhance overall safety and compliance.
For Regulatory Affairs Specialists
Regulatory Affairs Specialists are responsible for maintaining compliance with evolving regulatory requirements. Qualityze helps them stay current by providing real-time tracking of adverse events and compliance tools to streamline regulatory reporting, document submissions, and ensure alignment with FDA and global standards.
For Risk Managers
Risk Managers need to identify and mitigate risks associated with adverse events. Qualityze equips them with advanced analytics to monitor trends, assess risks, and take proactive measures to reduce liabilities and enhance patient safety. It enables a more structured approach to risk management, ensuring that all potential hazards are effectively addressed.
For Compliance Officers
Compliance Officers are responsible for regulatory adherence, and Qualityze helps them track and document all adverse event activities. With automated workflows, reporting tools, and audit-ready records, Compliance Officers can efficiently ensure compliance with FDA, ISO, and other relevant regulatory requirements.
For Pharmacovigilance Teams
Pharmacovigilance Teams are responsible for monitoring adverse drug reactions and ensuring drug safety. Qualityze helps them capture, evaluate, and report adverse events accurately, ensuring timely regulatory submissions and enhancing the overall safety profile of pharmaceutical products.
Experience the Qualityze Difference in Managing Adverse Events with a Free Demo
We understand that choosing the right Adverse Event Management solution is an important decision for your business. That's why we invite you to see Qualityze in action through a free demo. Here are three compelling reasons to take advantage of this offer:
See AI-Powered Adverse Event Management in Action
Discover how Qualityze simplifies adverse event management with AI-driven features. From capturing event details to conducting severity assessments and creating corrective actions, Qualityze reduces manual effort, minimizes compliance risks, and provides real-time insights to make adverse event management proactive and efficient.
Discover What Your Ideal Adverse Event Management System Should Look Like
Qualityze provides a comprehensive solution to adverse event management by integrating seamlessly with other quality processes, including CAPA, Complaint Management, and Change Management. With its secure cloud-based platform, Qualityze offers a unified approach to managing adverse events, helping organizations track, assess, and respond to events effectively while maintaining compliance. This holistic integration simplifies workflows and ensures all quality processes are connected for improved efficiency and transparency.
Is It Worth the Hype? Find Out for Yourself!
See how Qualityze strengthens your adverse event management processes and enhances overall business efficiency. Our platform is designed to make event assessment, response, and monitoring easier, providing you with confidence, value, and peace of mind. Experience the capabilities that set Qualityze apart as a leader in the world of quality.
Ready to see Qualityze in action?
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
How does Qualityze Adverse Event Management Software work?
Qualityze Adverse Event Management Software works by providing a cloud-based platform to capture, manage, and analyze adverse events efficiently. It streamlines workflows, helps lifesciences organizations comply with regulatory requirements, and ensures patient safety by reducing risks.
How does Qualityze help in identifying and managing adverse events?
Qualityze Adverse Event Management helps organizations identify potential risks by automating data collection and analysis. It enables structured evaluation of adverse events based on severity and regional regulatory requirements, ensuring timely identification and management.
Can Qualityze Adverse Event Management handle regulatory reporting?
Yes, Qualityze simplifies regulatory reporting by providing clear guidance and automation for generating and submitting regulatory reports in compliance with local and international standards, such as FDA and ISO. It also makes regulatory submissions more efficient and error-free.
How does Qualityze ensure compliance with regulatory standards?
Qualityze Adverse Event Management aligns with global standards like FDA 21 CFR Part 820 and ISO 13485. The software automates compliance processes, ensuring organizations meet regulatory obligations consistently and effectively.
What role does automation play in Qualityze Adverse Event Management?
Automation helps eliminate manual data entry, reduce errors, and ensure efficient data processing. Qualityze automates adverse event data collection, risk assessment, reporting, and follow-up actions, freeing up valuable time for quality teams.
How does Qualityze Adverse Event Management promote collaboration?
Qualityze includes features like integrated communication tools that allow team members and stakeholders to collaborate seamlessly. This helps ensure everyone is informed, leading to better decision-making and efficient handling of adverse events.
What are the benefits of using Qualityze Adverse Event Management for healthcare providers?
Lifesciences organizations benefit from Qualityze Adverse Event Management by gaining real-time visibility into adverse events, ensuring patient safety, maintaining regulatory compliance, reducing operational costs, and improving overall healthcare quality.
Can Qualityze integrate with FDA?
Yes, Qualityze Adverse Event Management seamlessly integrates with FDA eMDR system, automating adverse event submissions in HL7 ICSR XML format and providing real-time tracking of Ack1, Ack2, and Ack3 acknowledgments.
What kind of analytics capabilities does Qualityze Adverse Event Management provide?
Qualityze provides powerful analytics tools that help identify trends and patterns in adverse event data. This supports data-driven decision-making, allowing organizations to improve their processes and prevent future adverse events.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
© 2025 Qualityze™ | All rights reserved. | Privacy Policy

























