Nonconformance Management Software
Accelerate Nonconformance Processes for Improved Oversight
Reduce nonconformance management cycle times while increasing control and visibility over every quality issue reported. Our AI powered nonconformance software helps eliminate process inefficiencies by automating workflows, ensuring regulatory compliance, and providing real-time insights that empower your team to investigate and resolve issues faster.

Intelligent QAI Assistant to Simplify Nonconformance Investigations
Detect patterns and predict issues before they escalate with our QAI assistant that provides real-time insights and automates routine tasks for faster resolutions like root cause analysis, suggests corrective actions, and recommends next-steps to accelerate your nonconformance workflows, ensuring timely resolutions.

Save Administrative Load with Standardized NC Templates
Before any nonconformance event can be identified and managed, it's essential to have the right templates in place. Qualityze Nonconformance Management allows you to configure NC templates for standardized repeatable processes tailored to your unique business needs—whether for specific products, processes, systems, or workflows. These templates serve as a blueprint, ensuring consistency across all nonconformance events. It further helps to save on administrative time and load.

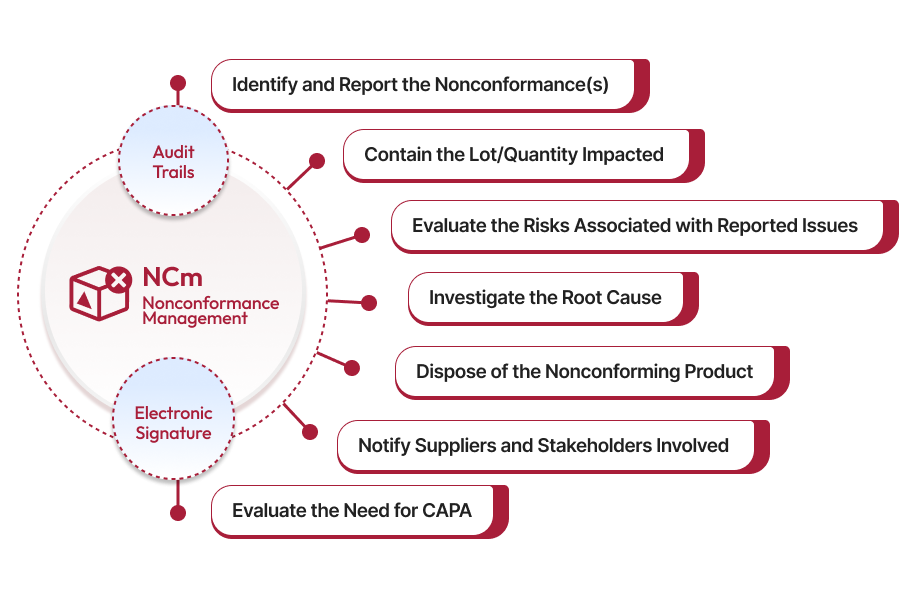
Ensure Timely Nonconformance Event(s) Reporting
Effective nonconformance management starts with timely identification and reporting of nonconformance events. Our robust solution enables you to efficiently capture every detail —including defect codes, root causes, process specifics, manufacturing, supplier, and customer information—into one centralized system. This proactive step not only ensures accountability but also demonstrates your commitment to maintaining high-quality standards.

Segregation for “Immediate Remediation”
After a nonconformance is reported, the Containment step ensures the issue is isolated to prevent further impact. This involves production delays, segregating defective products or processes, and implementing short-term corrective actions to minimize risks. The system comes with an in-built risk matrix. It generates a Risk Priority Number to help you with prioritizing the high-risk events without delays.

Minimize Risks with Rapid Disposal of Defective Products
The disposition step prevents compromised goods from reaching customers or going further into production. With Qualityze, you can document the lot numbers, quantities, and reasons for disposal to maintain compliance and ensure traceability. It facilitates collaboration with relevant teams and suppliers through instant notifications to ensure that every impacted product is properly accounted for, minimizing risks and maintaining the integrity of the supply chain.
Fast-Track Resolution with Root Cause Investigation
The next step is a thorough investigation to identify its root cause. This involves gathering data and using structures methods like 5 Whys, or 5 How's. Standardized defect codes and categorization help streamline the evaluation, ensuring that high risk severity issues are prioritized. Proper documentation of this phase is essential for compliance and future audits, aligning with industry best practices in nonconformance workflows.

Drive Continuous Improvements with Tailored Action Plans
After identifying the root cause, the action plan is designed to correct the issue and prevent recurrence. It assigns clear tasks to relevant teams with deadlines, ensuring prompt execution. Implementing the plan involves tracking progress and verifying effectiveness. Continuous monitoring and documentation ensure compliance and drive long-term improvements across the organization, resolving the nonconformance efficiently.

Escalate to CAPA for Proactive Quality Control
This step involves evaluating the severity and frequency of the problem to determine if corrective actions are required to address the immediate issue and preventive actions to mitigate future risks. CAPA ensures long-term solutions are implemented, preventing recurrence and enhancing overall process effectiveness. Proper documentation and analysis are critical to justify CAPA and maintain compliance with regulatory standards.
Do you know?
Loading random fact...
saf
COQ
We help you save on cost of quality!
What Differentiates Us from Others We have got you covered for everything you look for in a next generation nonconformance management solution to be future-ready!
- End-to-End Nonconformance Control
Unlike others nonconformance tracking systems, our solution offers complete end-to-end management of nonconformance events, from detection to resolution. With real-time tracking, integrated root cause analysis, and automated workflows, we ensure that every nonconformance issue is addressed with precision and speed.
- Configurable to Fit Your Needs
We understand that every business has unique processes. That’s why our platform is fully configurable, allowing you to tailor workflows, templates, and defect codes to align with your specific industry requirements—whether in manufacturing, healthcare, or beyond.
- Built-In Compliance Tools
Compliance is at the core of what we do. Our solution is designed to meet strict regulatory requirements like ISO, FDA, and GxP. We provide built-in compliance checks, audit-ready reporting, and traceable workflows, ensuring you're always prepared for inspections or audits.
- AI-Powered Insights
What sets us apart is our AI-driven intelligence. Our software not only tracks nonconformance events but also uses AI to predict trends, recommend corrective actions, and continuously improve processes, helping you stay ahead of potential issues.
- Seamless Integration
Integration is key in today’s digital landscape. Our platform seamlessly integrates with your existing systems (ERP, CRM, and more), ensuring a unified approach to quality management. This reduces data silos, increases visibility, and enhances collaboration across teams.
- User-Friendly Interface
While other systems may be complex and difficult to navigate, our platform offers an intuitive, user-friendly interface that requires minimal training. This ease of use ensures quick adoption by your team, reducing downtime and accelerating results.
- Continuous Improvement Culture
We not only help you fix problems; we help you prevent them. By providing actionable insights and tracking nonconformance trends, our software promotes a culture of continuous improvement, driving long-term operational excellence.
- Unparalleled Support and Training
What truly differentiates us is our commitment to your success. We offer ongoing training, personalized support, and regular updates to ensure that your nonconformance management processes are always optimized and aligned with industry best practices.
Industry Recognitions



Qualityze Delivering Value Across Key Roles
Discover how Qualityze Nonconformance Management empowers every key role in managing NCs for seamless collaboration and success —from quality managers to regulatory affair manager.
For Quality Manager
Qualityze gives you complete visibility and control over the nonconformance process, from identification to resolution. The software automates workflows, tracks corrective actions, and ensures compliance with industry standards like ISO and FDA. By streamlining nonconformance management, you can focus on driving continuous improvement while ensuring that quality and regulatory requirements are consistently met.
For Production Manager
With Qualityze, you can address production-related nonconformance issues without halting operations. The software provides real-time visibility into defects, automates root cause analysis, and implements corrective actions quickly. You’ll be able to minimize downtime, reduce rework, and ensure that production continues smoothly while maintaining high-quality standards.
For Quality Control/Quality Assurance (QC/QA) Technicians
Qualityze simplifies the process of identifying and documenting nonconformances during inspections or audits. With easy-to-use forms, defect categorization, and automated workflows, you can escalate issues quickly and ensure they’re resolved efficiently. The software empowers you to stay ahead of quality risks while reducing manual documentation tasks.
For Root Cause Analysis Specialists
Qualityze provides powerful tools like root cause analysis, defect code management, and integrated reporting, allowing you to investigate nonconformance events with precision. Whether using 5 Whys or Fishbone Diagrams, the software helps you get to the root cause faster, ensuring that corrective actions target the actual issue and prevent future occurrences.
For CAPA Coordinator
Qualityze enables you to easily manage and monitor all Corrective and Preventive Actions (CAPA). The software automates task assignments, tracks completion, and verifies the effectiveness of actions, ensuring full compliance with regulatory requirements. By streamlining CAPA workflows, you can ensure timely resolutions and drive continuous improvement initiatives.
For Regulatory Affairs Manager
Qualityze ensures that your nonconformance processes are always compliant with FDA, ISO, and other regulatory standards. The software provides audit-ready documentation, tracks corrective actions, and ensures that all nonconformance events are properly addressed. You can stay audit-prepared while minimizing the risk of regulatory fines or compliance issues.
Experience the Qualityze Difference to Manage Nonconformances with a free demo. Here’s what you’ll get to see in the demo:
We understand that choosing the right nonconformance software is a big decision. That’s why we invite you to experience Qualityze for yourself with a free demo. Here are three compelling reasons to take us up on this offer:
See AI-Powered Nonconformance Management in Action
Witness firsthand how our intelligent, AI-driven nonconformance management system automates tasks, identifies patterns, and streamlines processes—making your quality management faster and more efficient.
Discover What Your Perfect Nonconformance Tracking System Should Look Like
Explore how Qualityze advanced nonconformance tracking system provides real-time visibility, intuitive dashboards, and comprehensive reporting tools, giving you complete control over nonconformance events.
Is It Worth the Hype? Find Out for Yourself!
Don’t just take our word for it. Use the demo to see if Qualityze lives up to its reputation as a leader in the world of Quality. Experience how easy it is to ensure compliance, streamline workflows, and drive continuous improvement.
Ready to see Qualityze in action?
Request Demo
Frequently asked questions
Answers to commonly asked questions
If you have more questions feel free to reachout to us.
Contact UsGeneral
What is Nonconformance Management Software, and how does it help?
Nonconformance Management Software is a system that helps businesses detect, document, and resolve nonconformance events that deviate from quality standards. It automates workflows, tracks issues, and ensures compliance with regulatory standards, helping organizations resolve quality problems efficiently and prevent them from recurring.
How does Qualityze Nonconformance Management System work?
Qualityze offers a comprehensive nonconformance management system that automates the entire process, from detection and categorization to root cause analysis and corrective actions. It provides real-time tracking, automated workflows, and AI-driven insights to streamline nonconformance management and ensure regulatory compliance.
What is the difference between Corrective and Preventive Actions (CAPA) and Nonconformance Management?
While nonconformance management focuses on identifying and resolving quality issues that have already occurred, CAPA involves implementing corrective actions to fix the root cause of the issue and preventive actions to stop it from happening again in the future. Nonconformance management is often the starting point for initiating CAPA.
Why is defect code management important in a Non Conformance Tracking System?
Defect code management is critical in a non conformance tracking system because it standardizes how issues are categorized, making it easier to track recurring problems, analyze trends, and implement corrective actions. By using predefined or customizable defect codes, businesses can ensure consistent categorization and faster resolution of nonconformance events.
What industries benefit most from Nonconformance Management Software?
Nonconformance management software is widely used in industries with stringent quality and regulatory requirements, such as manufacturing, healthcare, pharmaceuticals, medical devices, automotive, and aerospace. These industries rely on nonconformance management to ensure product quality, safety, and compliance with standards like ISO and FDA regulations.
How does AI improve Nonconformance Management?
AI enhances nonconformance management by automating manual tasks, detecting patterns in nonconformance events, and predicting future quality risks. AI-powered insights help organizations resolve issues faster and prevent similar nonconformances from recurring. Qualityze AI-powered recommendations make it easier to identify root causes, implement corrective actions, and continuously improve quality management processes.
How can I ensure my Nonconformance Tracking System is compliant with regulatory standards?
A compliant nonconformance tracking system must align with industry regulations such as FDA, ISO, and GxP. It should provide features like audit-ready documentation, traceability, automated workflows, and reporting capabilities. Qualityze ensures compliance with built-in regulatory checks, documentation features, and audit trails.
What is the role of Root Cause Analysis in Nonconformance Management?
Root Cause Analysis is a crucial part of nonconformance management, helping to identify the underlying reasons for a nonconformance event. Tools like 5 Whys or Fishbone Diagrams are commonly used to determine why the issue occurred, ensuring that corrective actions target the real problem and preventing its recurrence.
How does Qualityze ensure real-time tracking in Nonconformance Management?
Qualityze provides a robust, cloud-based nonconformance tracking system that offers real-time visibility into nonconformance events. With customizable dashboards, automated notifications, and integrated reporting tools, Qualityze ensures that teams can track, manage, and resolve nonconformances in real-time, minimizing downtime and enhancing overall quality management.
Compliance
How Qualityze EQMS ensures compliance?
Qualityze EQMS ensures compliance by providing features that comply with industry-specific standards, such as ISO 9001, ISO 13485, 21 CFR Part 11, 21 CFR Part 820, AS9100, and IATF 16949. The solution is built on the Salesforce platform, which provides a secure and compliant cloud infrastructure. Qualityze EQMS allows businesses to maintain a complete audit trail of all quality data, ensuring regulatory compliance and reducing the risk of non-compliance.
The software also provides configurable workflows that allow businesses to enforce compliance with their own internal policies and procedures. The workflows can be customized to fit specific requirements and ensure that all processes are standardized and consistent across the organization. The solution provides real-time visibility and reporting, allowing businesses to quickly identify areas of non-compliance and take corrective action. Qualityze EQMS also provides automatic version control and document management features, ensuring that all documents are up-to-date and comply with relevant regulations.
How Qualityze can provide free life time updates? What does “free lifetime update" include?
As a cloud-based solution, Qualityze EQMS is continually updated with the latest features and functionalities to ensure compliance with changing regulations and industry standards. Qualityze provides free lifetime updates to its customers to keep them up-to-date with the latest technology advancements and features. These updates are automatically applied to the system without any additional cost or disruption to the user. Qualityze’s software as a service (SaaS) model allows for seamless integration of new features and bug fixes, which means that customers always have access to the latest version of the software. Qualityze’s dedicated team of developers and quality experts continuously work on enhancing the product to ensure that it meets the evolving needs of the customers and helps them stay ahead of the competition.
What does Qualityze's free lifetime update include?
Qualityze's free lifetime updates include bug fixes, security updates, feature enhancements, and new functionalities. These updates ensure that the Qualityze EQMS solution stays up-to-date and continues to meet the evolving needs of the industry and regulatory standards. The updates are provided without any additional cost to the customers and are automatically deployed to their systems. The Qualityze team ensures that the updates are thoroughly tested before being released to the customers to ensure their smooth integration and minimal disruption to their business operations. The free lifetime updates help customers to leverage the latest technological advancements and ensure that their quality management processes remain compliant and effective.
Security
- Data Encryption: Qualityze uses AES-256 encryption to secure data in transit and at rest, providing a high level of data security.
- Role-Based Access Control: Qualityze provides access controls based on user roles, ensuring that users only have access to the data they need to do their job.
- Two-Factor Authentication: Qualityze uses two-factor authentication to provide an additional layer of security when logging in to the system.
- Regular Security Audits: Qualityze conducts regular security audits to identify and address any vulnerabilities in the system.
- Compliance with Industry Standards: Qualityze complies with industry-specific standards such as ISO 9001, ISO 13485, 21 CFR Part 11, 21 CFR Part 820, AS9100, and IATF 16949, ensuring that the system meets the highest security standards.
How secured is Qualityze EQMS?
Qualityze EQMS provides a highly secure environment for managing quality data. It is built on the world’s leading cloud-based platform, Salesforce.com, which has a robust security framework with multiple layers of security features, including:
Overall, Qualityze EQMS provides a secure environment for managing quality data, giving organizations peace of mind that their sensitive data is protected.
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
© 2025 Qualityze™ | All rights reserved. | Privacy Policy

























