CAPA Management Solution
Proactive CAPA Management to Resolve Quality Issues at the Root
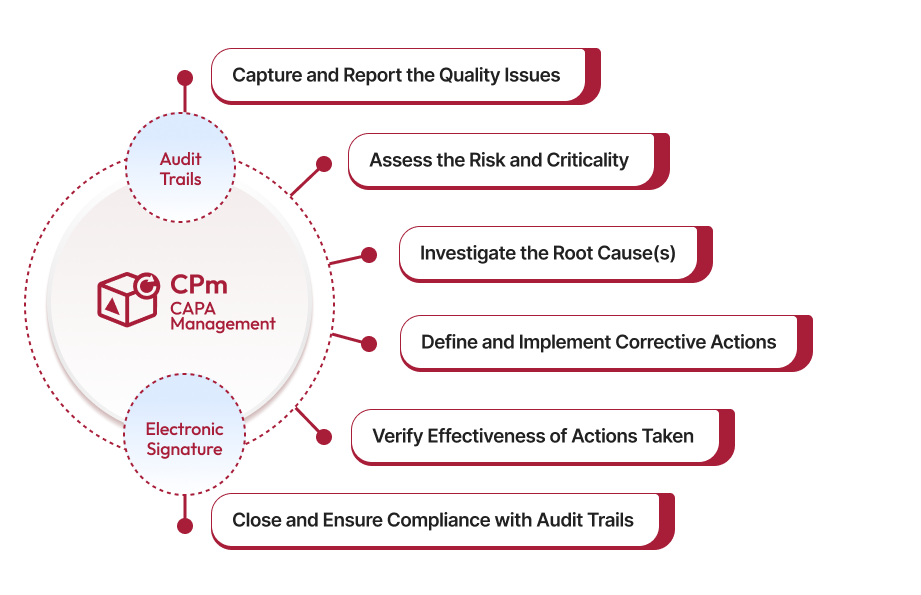
Regulated industries requires a consistent CAPA (Corrective and Preventive Actions) framework to eliminate systemic quality issues, from defects to nonconformances. Our AI-powered CAPA management system empowers businesses to align their workflows with proven best-practices and regulatory standards to minimize CAPAs and related risks.

Proactive Quality Assurance with AI – Your Intelligent QAI Assistant
Accelerate CAPA investigations, automate workflows, and get recommendations for corrective actions with our QAI assistant. Our solution provides real-time insights into potential risks, ensuring faster resolutions, while helping you maintain regulatory compliance, and drive continuous improvement.

Ensure Consistency Right from the Beginning
Save time and ensure data integrity with ready-to-use, pre-built CAPA workflows. You can capture all CAPA-related information in a centralized database. CAPA fields typically include the Title, Description, Problem Statement, CAPA Source, an Auto-generated Number, Reported Date, Reported By, Criticality, and CAPA Owner. You can easily add or remove fields to tailor the workflow to your specific requirements. Using standardized CAPA forms helps maintain consistency and accuracy in reporting, analysis, and root cause investigation.

Detailed Reporting to Address Systemic Issues Faster
Our system captures every detail needed to resolve issues quickly and effectively. You can track root causes, assign responsibilities, and keep everyone in the loop with just a few clicks—ensuring accountability and transparency. With built-in reporting features, you can generate detailed reports on the status of corrective actions, driving accountability and ensuring timely execution of corrective actions. This clarity drives faster, more informed decision making.

Prioritize the Right Issues with Risk Assessment Tools
Some problems require immediate attention! Our built-in Risk Matrix helps you to identify those problems by assessing each issue's potential impact. The system helps evaluate risk of the CAPAs reported so your team can prioritize their tasks accordingly. This strategic prioritization helps minimize downtime and prevent the risk of recurring quality issues.

Get to the Root Cause with Structured Investigations
Uncover the root cause. Our CAPA solution comes with tools like 5 Whys, 5 How's, fishbone and more to help you uncover the real cause of problems, so you can resolve them effectively. Our in-built investigation tools help your team take a thorough and systematic approach to mitigate underlying issues. It further helps you to develop and implement effective action plans to prevent their recurrence, ensuring proactive risk management.

Automate Workflows to Keep Things Moving
Set up automated reminders, email alerts, and schedule tasks in advance to make sure nothing slips through the cracks. With clear deadlines and smooth handoffs, your team will stay focused and productive. Automation also reduces manual tasks, allowing your team to focus on more strategic activities. This reduces the risk of delays and achieves faster resolution of CAPAs.

Check Effectiveness to Ensure Long-Term Success
It’s not enough to fix the problem—you need to know whether the implemented action plans are effective, reducing the likelihood of recurring issues. Our solution makes it easy to measure the desired result of the corrective actions and, make adjustments as needed. By validating the success of corrective actions, you can maintain compliance and boost confidence in your quality management processes.
Industry Recognitions



Qualityze Delivering Value Across Key Roles
Discover how Qualityze CAPA Management solution enables seamless collaboration between quality, regulatory, and operations teams ensuring efficient and effective CAPA resolution.
For Quality Managers
Gain full control over your CAPA processes from the initial documenting stage till resolution. With Qualityze, you can focus on identifying improvement opportunities, minimizing recurring issues, and proactively driving continuous quality enhancements. By automating routine tasks, our solution helps minimize errors and maintain compliance with industry standards like ISO and FDA—allowing you to focus on strategic quality initiatives that matter most.
For Production Managers
Ensure seamless operations without disruption by addressing production issues swiftly and effectively. Qualityze enables you to trace and resolve root causes quickly, preventing issues from escalating. Our solution minimizes downtime by integrating corrective actions into production workflows, so you can fix problems without slowing operations. Our QAI assistant shares actionable insights from historical data to fast-track decision-making processes
For QA/QC Technicians
Streamline the way you capture, log, and manage CAPA-related issues during inspections, audits, or production runs. Qualityze provides user-friendly forms, automated workflows, and centralized data entry, ensuring every issue is documented accurately and efficiently. Our system makes reporting fast and hassle-free, giving QA/QC teams the confidence to focus on improving quality standards and staying compliant during external audits or inspections.
For Root Cause Specialists
Investigate and resolve nonconformance events with precision using Qualityze’s advanced diagnostic tools, including 5 Whys, 5 Hows and Fishbone Diagrams. Our platform empowers you to dig deep into problems to uncover the true root causes, allowing for meaningful and effective corrective actions. Implement preventive actions on time to eliminate recurring issues and ensure that your organization stays clear of potential risks and future nonconformances.
For CAPA Coordinators
Simplify CAPA management by effortlessly assigning tasks, tracking progress, and keeping all stakeholders aligned and informed with real-time notifications and automated reminders. With Qualityze, you can ensure that every corrective and preventive action is properly executed and closed on time. The platform helps you avoid bottlenecks by maintaining full transparency throughout the process—ensuring every step is monitored, documented, and completed efficiently.
For Regulatory Affairs Managers
Maintain audit readiness with Qualityze traceable workflows and comprehensive reporting tools that align with regulatory frameworks like ISO, FDA, and other global standards. Our system ensures that every event is properly documented, with evidence-backed corrective actions that meet compliance requirements. With Qualityze, you can easily generate reports for inspections, reducing the burden of audits and giving your team confidence and peace of mind throughout the process.
Experience the Qualityze Difference to Manage CAPAs with a free demo. Here’s what you’ll get to see in the demo:
We understand that choosing the right CAPA solution is a big decision. That’s why we invite you to experience Qualityze for yourself with a free demo. Here are three compelling reasons to take us up on this offer:
Experience AI-Powered CAPA Management Like Never Before
See how our intelligent, AI-driven CAPA management system automates repetitive tasks, uncovers actionable insights, and optimizes processes—delivering faster, smarter quality management at every step.
Discover the Ideal CAPA Management System for Your Organization
Explore the Qualityze difference through our advanced CAPA solution, featuring real-time visibility, intuitive dashboards, and robust reporting tools. It helps you go reactive to proactive risk management to ensure minimum to zero chances of recurrences.
Is It Worth the Hype? Find Out for Yourself!
Don’t just take our word for it. Schedule a free demo to see if Qualityze lives up to its reputation as a leader in the world of Quality. Experience how easy it is to ensure compliance, streamline workflows, and drive continuous improvement.
Ready to see Qualityze in action?
Request Demo
Products
All Qualityze ProductsNonconformance ManagementCAPA ManagementDocument ManagementChange ManagementTraining ManagementAudit ManagementSupplier Quality ManagementComplaints ManagementCalibration ManagementMaintenance ManagementInspection ManagementPermit ManagementMaterial Compliance ManagementForms ManagementField Safety & Recall ManagementAdverse Events ManagementIncident ManagementRisk ManagementBatch Records Management8D Process
Industries
LifesciencesFood & BeveragesHealthcareManufacturingMedical DevicesPharmaceuticalsBiologicsBiotechnologyNutraceuticalsCannabisCompounded DrugsBlood & TissueAutomotiveAerospace & DefenseElectric VehiclePlastic and RubberElectronics and AppliancesChemical & AgrochemicalOil & GasEnergy & UtilitiesMetals & Mining
© 2025 Qualityze™ | All rights reserved. | Privacy Policy